
Upgrade to High-Speed Internet for only ₱1499/month!
Enjoy up to 100 Mbps fiber broadband, perfect for browsing, streaming, and gaming.
Visit Suniway.ph to learn
LONDON (AP) — The European Medicines Agency has recommended authorizing a twice-yearly injectable drug aimed at preventing HIV, which scientists say could help end the virus' transmission.
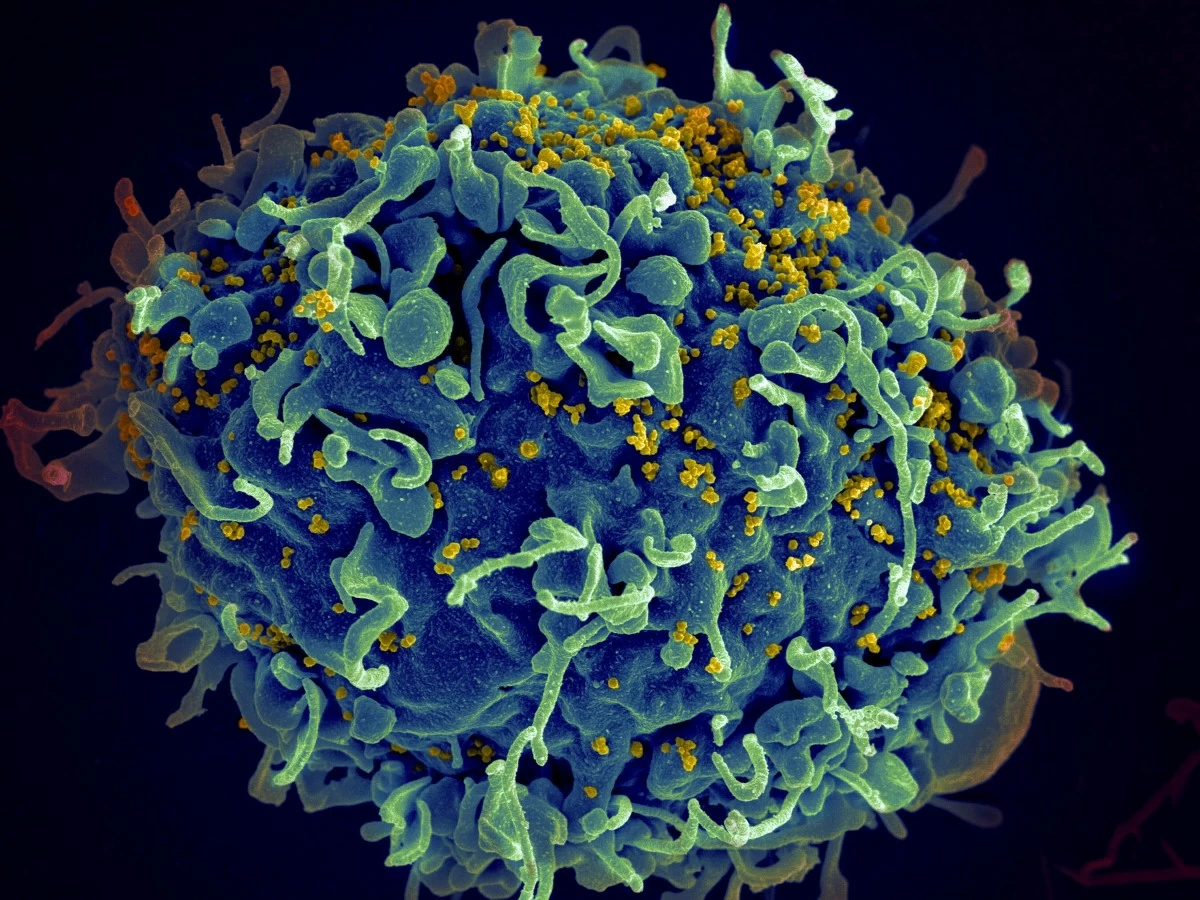
FILE - This colorized electron microscope image provided by the U.S. National Institutes of Health shows a human T cell, in blue, under attack by HIV, in yellow, the virus that causes AIDS. (Seth Pincus, Elizabeth Fischer, Austin Athman/National Institute of Allergy and Infectious Diseases/NIH via AP, File)
In a statement on Friday, the EU drug regulator said its evaluations of lenacapavir, sold as Yeytuo in Europe by Gilead Sciences, showed the drug is "highly effective" and "considered to be of major public health interest." Once the regulator's guidance is accepted by the European Commission, the authorization is valid in all 27 EU member countries as well as Iceland, Norway and Liechtenstein.
Last year, studies suggested that lenacapavir, already used to treat people with HIV, was nearly 100% effective in stopping transmission in both women and men.
Winnie Byanyima, executive director of the U.N. AIDS agency, has said the drug "could change the trajectory of the HIV epidemic" if it is made available to everyone who needs it.
In June, the U.S. Food and Drug Administration authorized lenacapavir to prevent HIV. Earlier this month, the World Health Organization recommended countries offer the drug as an additional option to people at risk of the virus.
Condoms help guard against HIV infection if used properly. Other medication aimed at preventing HIV include daily pills that people can take and another injectable drug called cabotegravir, which is given every two months. Lenacapavir's six-month protection makes it the longest-lasting type, an option that could attract people wary of more visits to health clinics or stigma from taking daily pills.
Critics have raised concerns, however, that lenacapavir may not be made widely enough available to stop global outbreaks of HIV. Drugmaker Gilead has said it will allow cheap, generic versions to be sold in 120 poor countries with high HIV rates — mostly in Africa, Southeast Asia and the Caribbean.
But it has excluded nearly all of Latin America, where rates are far lower but increasing, sparking concern the world is missing a critical opportunity to stop the disease.
Last year, there were about 630,000 AIDS deaths worldwide and more than 40 million people are estimated to have HIV, according to UNAIDS.
UNAIDS chief Byanyima has previously suggested the U.S. President Donald Trump make a deal with Gilead to produce and license its "magical" prevention drug lenacapavir across the world to the millions of people who need it.

 17 hours ago
2
17 hours ago
2



